





InteliSwab COVID-19 Rapid Test - OTC Home Test Kit
|
On Clearance. Expired 10/3/2022, but please note, the FDA has extended the product expiration to 12 months for InteliSwab® COVID-19 Rapid Tests. Orders for InteliSwab COVID-19 Rapid Test are non-cancelable, non-returnable, and non-refundable. Please contact us if you have further questions. |
Authorized for over-the-counter use, this Rapid Test by OraSure Technologies has with no confusing steps, no batteries and no mailing to labs. Just easy-to-use convenience that makes it easier for individuals with or without symptoms to test themselves anytime, anywhere, and take all the necessary precautions if they test positive.
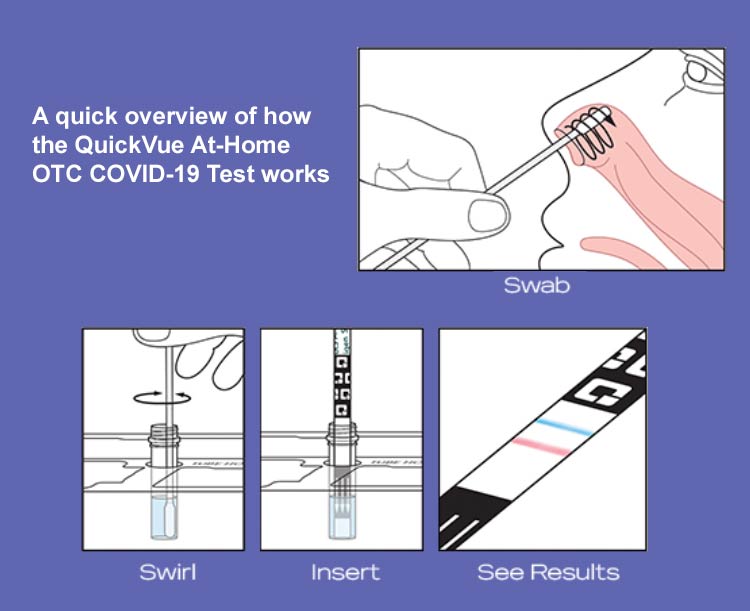
InteliSwab TM COVID-19 Rapid Test can meet the needs of any employer, university, physician office, public health testing site or venue large or small. All you would need to do is 3 simple steps:
- swab your nostrils with the gentle swab,
- swirl it in the tube, and
- see results in minutes.
It’s just that simple.
COVID-19 Update: On Clearance.
Model No. 1001-0616: By Box (2 tests) or 4 Boxes (8 tests)
This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA.
This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
The FDA has extended the product expiration to 12 months for InteliSwab® COVID-19 Rapid Tests.
Instructions for Use (English)
Related Article: InteliSwab COVID-19 Rapid Test For In-Home COVID-19 Testing